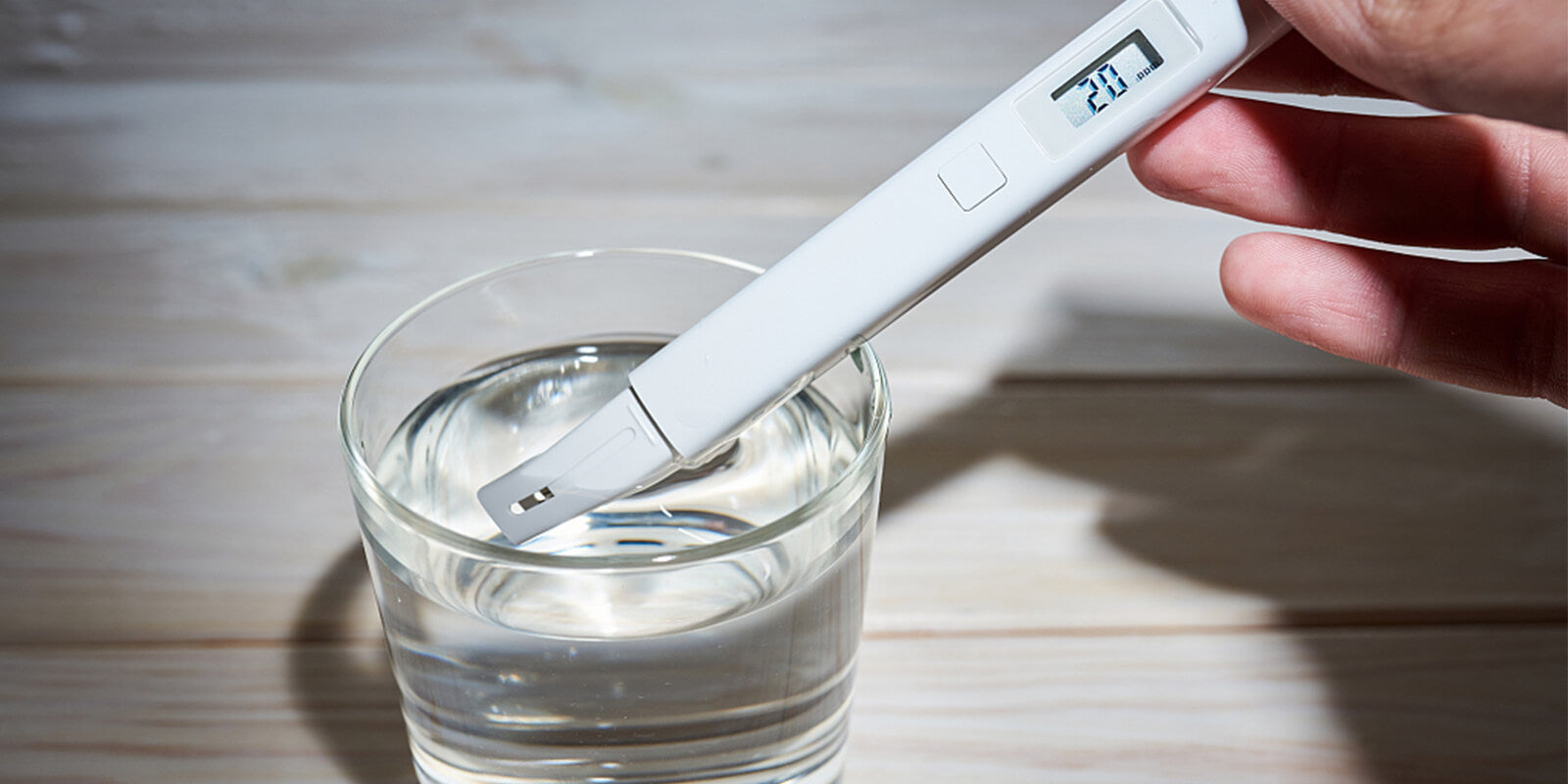
All sources of natural water include dissolved substances and minerals. These minerals are measured as the total dissolved solids or TDS. It is comprised of natural minerals, which are inorganic salts. Some of these are potassium, calcium, magnesium, chlorides, bicarbonates, and sulfates. In addition, it can have contaminants like heavy metals, which are low in concentrations. When you measure the TDS, the presence of heavy metals cannot be exactly determined.

In the water, TDS is identified as parts per million (ppm) or mg/L. Since TDS does not risk people's health, so it suggests for the drinking water to have a level of up to 500 ppm only.
If the water has above 500 ppm, you can notice deposits on the water, salty taste, or staining. These effects are not harmful. However, it can be noticeable.
The TDS in drinking water comes from natural water sources, sewage, urban run-off, industrial wastewater and chemicals used in the water treatment process, and the hardware or piping used to distribute water. Higher TDS was brought by natural environment features like salt deposits, mineral springs, seawater intrusion, and carbonate deposits in the US.
Other sources may include anti-skid materials, salts used for road de-icing, stormwater, agricultural runoff, water treatment chemicals, and point/non-point wastewater discharges.
In general, the total dissolved solids concentration is the total cations (positively charged) and anions (negatively charged) ions in the water.
The following table can be used to generalize the relationship of TDS to water quality problems.
| Cations combined with Carbonates. | Associated with hardness, scale formation, bitter taste |
| CaCO3, MgCO3, etc | |
| Cations combined with Chloride |
Salty or brackish taste increase corrosivity |
| NaCl, KCl |